The ability to use low energy visible light instead of uv in the photochemical activation of azides avoids competitive photodecomposition processes that have long been a significant limitation.
Formation of vinyl azides.
A practical rapid and efficient microwave mw promoted nucleophilic substitution of alkyl halides or tosylates with alkali azides thiocyanates or sulfinates in aqueous media tolerates various reactive functional groups.
Treatment of o deuterated aryl vinyl azide 1 o d 1 with tba fe led to a clean formation of indole 3 o d h in 70 yield.
28 the reaction is thought to proceed through the addition of manganese iii enolate 91 to vinyl azide 19 via a radical pathway giving iminyl radical 92 with the release of a.
Recently chiba and narasaka demonstrated mn iii catalyzed pyrrole formation from variously substituted vinyl azides and β keto esters or 1 3 diketones scheme 16.
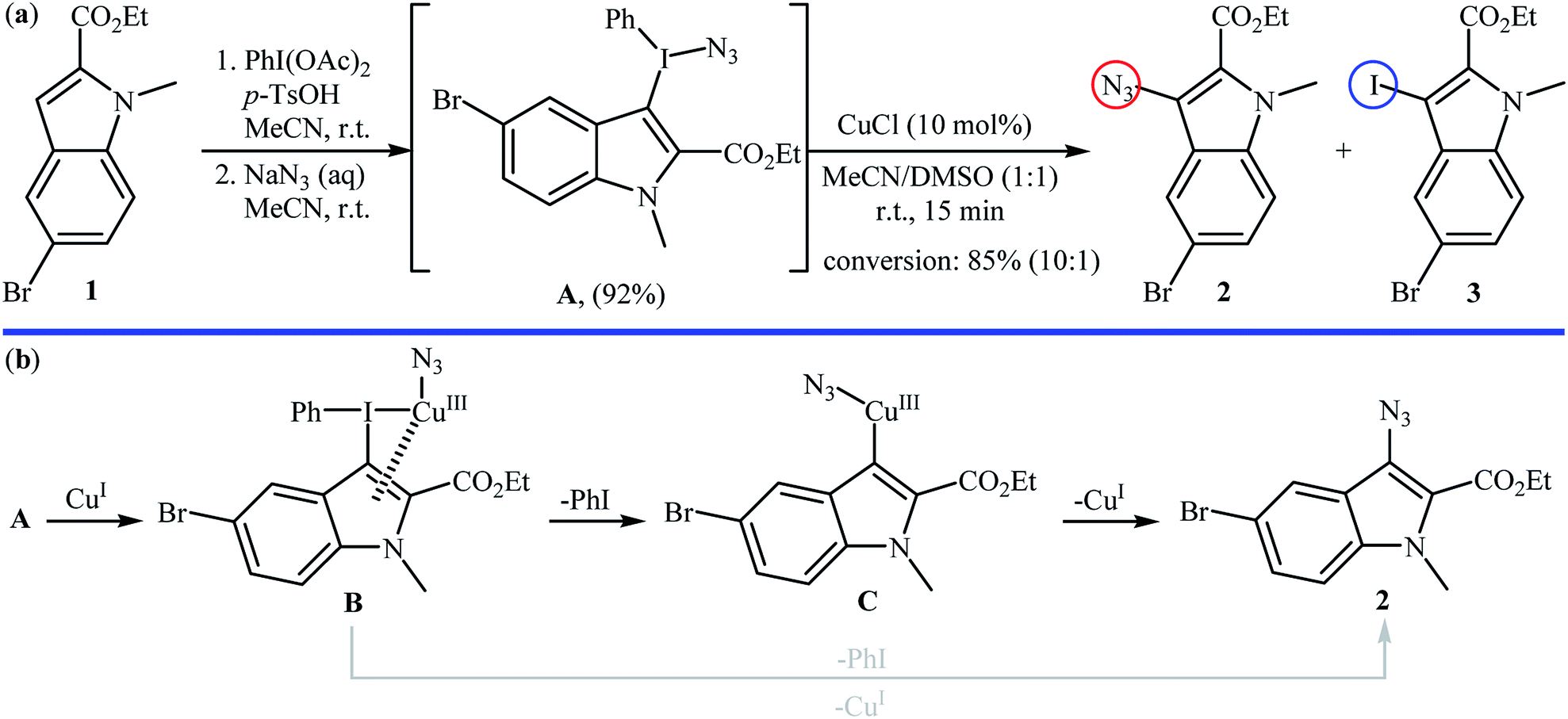
Among organic azides vinyl azides have shown versatile chemical reactivities in the recent development of new synthetic methodologies mainly for nitrogen containing molecules.
Reaction of allenyl esters with sodium azide.
These information suggest that we can exclude the in.
Hi or t r 3 2 r rhydrogen alkyl aryl 2 r ralkyl aryl in the case ofterminal vinyl azides several workers assumed 1zirine as.
An important one being azide is also a functional group in organic chemistry rn 3.
C n bond formation synthesis of azides synthesis of alkyl azides.
The journal of organic chemistry 2007 72 4 1534 1537.
C n bond formation synthesis of azides synthesis of vinyl azides.
Azide is the anion with the formula n 3 it is the conjugate base of hydrazoic acid hn 3 n 3 is a linear anion that is isoelectronic with co 2 nco n 2 o no 2 and ncf per valence bond theory azide can be described by several resonance structures.
The requisite 3 azido e vinyl sulfones were prepared from 3 bromo e vinyl sulfones which in turn were accessed from allyl sulfones via a bromination elimination sequence.
An efficient synthesis of e vinyl azides and polysubstituted pyrroles.
Irradiation of vinyl and aryl azides with visible light in the presence of ru photocatalysts results in the formation of reactive nitrenes which can undergo a variety of c n bond forming reactions.
The dominant application of.
This synopsis highlights and discusses recent advances on use of vinyl azides in chemical synthesis as a radical acceptor and an enamine type nucleophile.
A small kinetic isotope effect was observed indicating that the c h bond scission process might not be the rate limiting step equ.